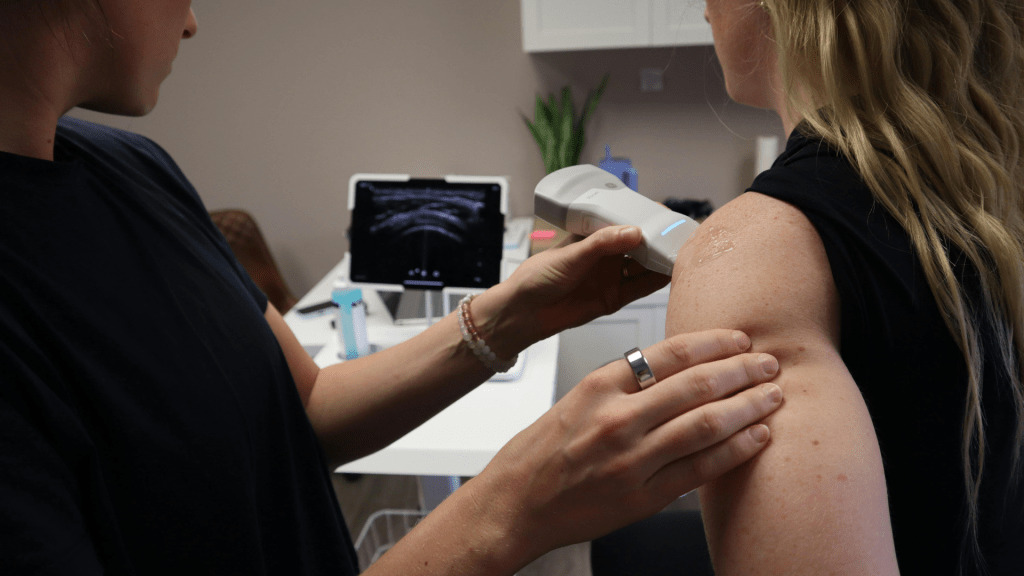
Consulting Professionals
At Qualisure Consulting Ltd, our team of industry experts brings unparalleled experience and dedication to every project.
We specialise in innovative strategies that transform businesses and drive success.

20 years of medical device quality and regulatory excellence

Comprehensive QMS auditing and regulatory compliance support for the medtech sector

Accessible, affordable and effective quality and regulatory training services

Expert Medical Device Regulatory Affairs & Quality Management Consulting
Qualisure Consulting delivers specialist regulatory affairs and quality management system (QMS) consulting services for medical device manufacturers navigating UK MDR, EU MDR 2017/745, and IVDR 2017/746 compliance.
We transform complex regulatory challenges into streamlined pathways to market authorization and ISO 13485 certification.

QMS Auditing
Comprehensive internal audits to ISO 13485 and ISO 9001 standards, identifying gaps and supporting continuous improvement.

Regulatory Services
End-to-end support for technical documentation and risk management, including MDR/IVDR, compliance, preparation and review.

MDSAP Preparation
Guidance and support for Medical Device Single Audit Program readiness, tailored for 1-2 or multiple jurisdictions.

Regulatory Training
Online, modular workshops covering ISO 13485, ISO 14971, MDR, FDA regulations, and the use of AI in regulatory affairs.

Thank you for a comprehensive audit. We greatly value your experience and insight, and your internal audits have become a key pillar of the QMS improvements through 2025
Head Diagnostics